Lupin India Limited| Products, Subsidiaries, and Revenue- 2024

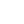
In 1968, a bold dream turned into reality! Dr. Desh Bandhu Gupta, affectionately known as DBG, borrowed ₹5000 from his wife, Manju Gupta, and founded Lupin India Limited. Driven by a mission to make a meaningful impact on health, he set out to create a company. Who knew that Lupin India would serve over 100 countries one day?
Curious to know what inspired the name ‘Lupin’?
Let’s look at the history of Lupin India Limited and see how it evolved into a global player in the pharmaceutical industry!
History of Lupin India Limited: Key Milestones, Products, and Global Expansion
Dr Desh Bandhu named the company after the Lupin flower, a symbol of resilience and nourishment. Just like the flower that thrives even in harsh conditions and enriches the soil, DBG wanted Lupin to sustain and contribute to society.
DBG’s journey began modestly.
He kickstarted a small setup with only two employees—a peon-cum-packer and a part-time typist. But what Lupin lacked in size, it made up for in spirit.
DBG’s vision was clear: improve public health in India. Lupin started by manufacturing vitamins.
However, he soon expanded to produce essential anti-TB drugs.
Do you know?
Lupin India was launched on Gudi Padwa, an auspicious festival in India.
Soon after, Lupin received its first major order from the Government of India. It had to supply iron and folic acid tablets for vital healthcare programs aimed at improving mother and child health.
So, from those early days with just two employees to a global presence today, Lupin’s journey is a remarkable growth story!
The journey was marked by significant milestones.
In 1972, Lupin set up its first formulations plant and R&D center in Aurangabad, India. By 1979, the company had advanced with the commissioning of a new formulations plant.
In 1981, it started the production of Ethambutol. This was an advancement in their portfolio.
1987 was a notable year with the opening of the Cephalexin plant at Mandideep and 7 ADCA plants at Ankleshwar, India.
This period saw rapid technological advancements and expansion.
Dr. Desh Bandhu Gupta established the Lupin Human Welfare and Research Foundation (LHWRF) in 1988. This was special because it happened long before CSR became popular.
In 1989, Lupin achieved global recognition with US FDA approvals for its Ankleshwar and Mandideep plants. The company also set up Lupin Chemicals (Thailand) Ltd. through a joint venture.
1991 saw the start of injectable Cephalosporin production at Mandideep, India.
By 1992, Lupin had set up a fermentation plant at Tarapur and a sterile plant for injectable Cephalosporins at Mandideep.
These are reflected in Lupin India’s growing capabilities.
The 1993 IPOs for Lupin Laboratories Ltd. and Lupin Chemicals Ltd. opened!
1997 saw approvals for 7 ACCA plants at Ankleshwar and the Rifampicin plant at Tarapur.
The company was all set for global expansion!
In 1999, it saw further global expansion with UK MCA approval for the Injectable Cephalosporin bulk active plant at Mandideep.
By 2000, Lupin achieved a significant milestone with US FDA approval.
2001 was a lucky year!
Lupin became the only Asian pharmaceutical company to receive US FDA approvals for its sterile Cephalosporin facility. The company also rebranded as Lupin Ltd. and commissioned a new oral Cephalosporin bulk active plant.
In 2002, Lupin’s patent filings also crossed 100.
In 2003, Lupin Pharmaceuticals Inc. was formed in the USA for trading and development.
2004 was notable for the launch of Suprax® in the US, marking Lupin’s entry into the US brands market.
2006 brought financial milestones in the history of Lupin.
A maiden issue of Foreign Currency Convertible Bonds (FCCB) and US FDA and MHRA approvals for its Goa facility.
The company issued bonus shares for the first time.
By 2007, Lupin had initiated commercial production at its dosage facility in Jammu, acquired Rubamin Laboratories Ltd. (renamed Novodigm Ltd.), and acquired Kyowa Pharmaceutical Industry Co. Ltd. in Japan.
In 2008, Lupin also acquired Hormosan Pharma GmbH, Germany, and established a biotech facility at Pune, India.
In 2009, it took over a majority stake in Multi-Care Pharmaceuticals Inc., Philippines.
By 2010, Lupin had become the fifth-largest generic player in the US!
In 2011, Lupin entered into a joint development agreement with Medicis Pharmaceutical Corporation, acquired worldwide rights for the Goanna® Brand and I’rom Pharmaceuticals, Japan, and began commercial production at a new oral solid dosage facility at Pithampur, India.
2012 saw Lupin’s inclusion in the NIFTY 50 Index!
In 2013, Lupin expanded its branded offerings by acquiring exclusive US rights for Alinia® oral suspensions.
2014 was a year of strategic expansion!
Lupin entered the Latin American market through the acquisition of Laboratorios Grin S.A. De C.V., Mexico.
It made way to complex injectables space with the acquisition of Nanomi B.V., Netherlands.
In 2015, Lupin opened the Center of Excellence in Florida.
In 2016, Lupin started a new plant in Japan. They also enhanced their US portfolio and acquired Shionogi & Co. and Gavis Pharma. In 2017, Lupin acquired Symbiomix Therapeutics and launched Softovac® in India. They opened another plant in Sikkim.
In 2019, Lupin partnered with Boehringer Ingelheim for a MEK inhibitor. They divested the Kyowa Pharmaceutical Industry.
2020 was notable for launching gProAir (Albuterol Sulphate), an essential inhalation product!
In 2023, Lupin launched its first inhalation product in Germany.
The company continues to expand with new brands, therapies, and digital solutions.
As Lupin continues its growth in 2024, it has just received tentative approval from the US FDA for its Emtricitabine and Tenofovir Alafenamide Tablets.
This breakthrough will see the tablets produced at Lupin’s state-of-the-art facility in Nagpur, India.
Thus, Dr. Desh’s 1968 vision continues to thrive today as well, all over 100 countries!
About Lupin India Limited
Lupin is the 7th largest pharmaceutical company in India. As of August 1, 2024, it has a market cap of ₹88283Cr. From branded and generic formulations to Active Pharmaceutical Ingredients (APIs), Lupin offers is all. The company is actively involved in groundbreaking drug discovery and development.
Their recent active research areas include:
Oncology (cancer treatment)
Immunology (immune system disorders)
Metabolic disorders (like diabetes).
Lupin India Limited is actively working on gynaecological issues, cardiovascular diseases, diabetes, asthma, pediatric health, central nervous system disorders, gastrointestinal problems, infections, and inflammation.
Lupin’s story is one of growth, innovation, and a steadfast commitment to making a difference in the world of healthcare.
Lupin India Products: Manufactures and Serves 1000+ products.
Lupin Limited Subsidiaries: 35, spead globally.
| Lupin Pharmaceuticals, Inc., USA, Kyowa Pharmaceutical Industry Co., Ltd., Japan, Pharma Dynamics (Proprietary) Ltd., South Africa, Hormosan Pharma GmbH, Germany, Multicare Pharmaceuticals Philippines, Inc., Philippines, Generic Health Pty Ltd., Australia, Kyowa CritiCare Co., Ltd., Japan, Nanomi B.V., Netherlands, Lupin Atlantis Holdings SA, Switzerland, Lupin Healthcare (UK) Ltd (formerly Lupin (Europe) Limited, UK), Lupin Australia Pty Ltd., Australia, Lupin Pharma Canada Ltd., Canada, Lupin Mexico S.A. de C.V., Mexico, Bellwether Pharma Pty Ltd., Australia, Lupin Philippines, Inc., Philippines, Lupin Diagnostics Limited, India, Generic Health SDN. BHD., Malaysia, Lupin Middle East FZ-LLC, Dubai, Lupin Inc., USA, Lupin GmbH, Switzerland, Nanomi B.V., Netherlands, Laboratorios Grin, S.A. de C.V., Mexico, Medquimica Industria Farmaceutica LTDA, Brazil, Gavis Pharmaceuticals, LLC, USA, Novel Laboratories, Inc., USA, Lupin Research Inc., USA, Lupin Pharma LLC, Russia, Lupin Ukraine LLC, Ukraine, Lupin Latam Inc., USA, Lupin Japan & Asia Pacific K.K., Japan, Lupin Management, Inc., USA, Symbiomix Therapeutics, LLC, USA, Lupin Europe GmbH, Germany, YL Biologics Ltd., Japan, Lupin Biologics Limited, India, Lupin Oncology Inc., USA, Lupin Digital Health Limited, India, Lupin Foundation, Avenue Coral Springs, LLC, Southern Cross Pharma Pty Ltd., Australia, Lupin Manufacturing Solutions Limited, Lupin Life Sciences Limited, Medisol S.A.S., France, Lymed S.A.S., France |
Lupin Revenue: For FY 2024, sales of formulations in India reached INR 66,564 million. This reflects a 9.6% increase from INR 60,759 million in FY 2023.
Total Number of Manufacturing Sites/Plants: Lupin India has 15 manufacturing facilities spread across India, the U.S., Brazil and Mexico.
Lupin Plants in India are located at the following places:
- Aurangabad, Maharashtra, India
- Ankleshwar, Gujarat, India
- Mandideep, Madhya Pradesh, India
- Pune, Maharashtra, India
- Tarapur, Maharashtra, India
- Goa, India
- Jammu, Jammu & Kashmir, India
- Vadodara, Gujarat, India
- Indore, Madhya Pradesh, India
- Nagpur, Maharashtra, India
- Visakhapatnam, Andhra Pradesh, India
- Sikkim, India
Lupin’s 25-Year Commitment: Empowering Communities and Sustaining the Environment
At Lupin, Corporate Social Responsibility (CSR) is viewed as more than just a legal requirement—it is a moral commitment. For over 25 years, the company has been dedicated to rural development through its foundation.
- The company works to empower underprivileged communities. They offer essential infrastructure and support environmental sustainability.
Their approach includes managing natural resources and promoting economic, social, and infrastructure development.
Lupin’s employees volunteer thousands of hours to social causes!
2. Lupin was among the first companies to join the global fight against COVID-19.
All CSR initiatives are managed through the Lupin Human Welfare and Research Foundation (LHWRF).
You may also want to know the History of Granules India Limited
FAQs| Lupin India Limited
Source- lupin.com, screener.in
______________________________________________________________________________________
Disclaimer: Investments in the securities market are subject to market risks; read all the related documents carefully before investing.